Raman spectroscopy sheds light on ancient chars, caves and the Notre Dame cathedral
Scientists in France have been studying charred material from archaeological sites with Raman spectroscopy. They showed that it is possible to determine what material was charred and at what temperatures. After the fire at Notre Dame cathedral in Paris, France in 2019, the researchers applied this method to determine the highest temperatures reached in the roof structure. In addition, they analysed chars from the Bruniquel caves in Southern France to show either animal or vegetal precursors.
Why study archaeological chars with Raman spectroscopy?
D. Deldicque and and J.-N. Rouzaud at Laboratoire de Géologie de l'Ecole Normale Supérieure de Paris in France have been studying chars to measure the highest carbonisation temperatures. At high temperatures and in the absence of oxygen, organic materials carbonise to form chars that contain polyaromatic layers. The growth of these layers is irreversible and depends on the carbonisation temperature. Raman spectroscopy is sensitive to the degree of carbonisation. The researchers describe this method as Raman paleothermometryi.

D. Deldicque of Laboratoiré de Géologie de Ecole Normale Supérieure de Paris uses an inVia™ confocal Raman microscope for his research on chars.
A highly sensitive inVia™ confocal Raman microscope recorded the Raman spectra from various chars. The spectra contain the two main carbon bands, called D and G bands, at around 1350 cm−1 and 1590 cm−1, respectively. The G band is associated with the vibrational modes of the sp2 hybridised carbons of the aromatic rings, and the D band is associated with the vibrational modes of the aromatic ring edges.
Raman spectra are sensitive to the growth of polyaromatic layers in chars. Higher temperatures result in a more intense carbon D band at 1350 cm-1. The height ratio HD/HG increases monotonically with heat-treatment temperatures up to 1300 ˚C. Using a calibrated curve, this method can determine the temperature of carbonisation with an accuracy of ±20 ˚C. The HD/HG ratio is therefore a suitable paleothermometer or a “fossil thermocouple.”
Measuring the hottest temperatures in the roof of Notre Dame de Paris during the fire of 2019
The Notre Dame de Paris Cathedral is a historical landmark dating from the Middle Ages. On 15th April 2019, a fire destroyed most of its 12th century oak framework. Notre Dame's iconic neo-Gothic spire collapsed. In contrast, the limestone structure mostly survived.
To aid the ongoing reconstruction of Notre Dame, it was important to determine the highest temperatures reached during the fire. Higher temperatures may have vaporised the lead from the cathedral roof and been a public health concern in the surrounding area. In addition, the fire may have weakened the remaining limestone masonry. Raman paleothermometry is the only method that can estimate the maximum temperatures experienced by the framework and vaults of Notre Dame.
Deldicque and Rouzaud applied Raman paleothermometry to charcoals collected after the fireii. First, they carbonised unburnt oak pieces from the cathedral to obtain a calibration curve between 500 ˚C to 1300 ˚C. Next, they validated the calibration using experimental fires equipped with thermocouples. Raman paleothermometry produced temperatures that agree with the direct measurements.
The researchers analysed charcoal samples from the transept, nave and crossing of the cathedral. The charcoals from Notre-Dame Cathedral, the charcoals from the crossing recorded the highest maximum temperature of 1200 ˚C. In the nave and in the northern transept, the highest maximum temperatures were 1088 ˚C and 1105 ˚C, respectively.

Carbonised beams under the nave of Notre Dame (photo courtesy of Damien Deldicque and Jean-Noël Rouzaud).
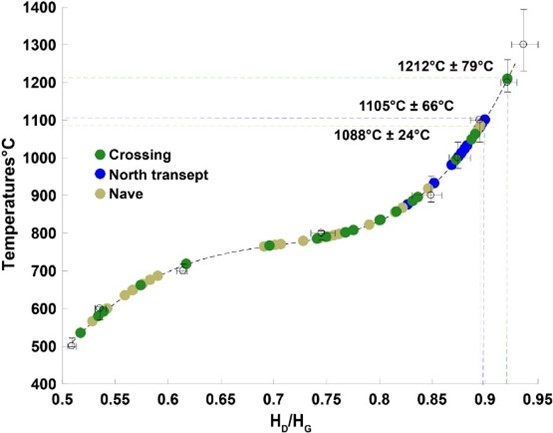
Retrieved paleotemperatures from the HD/HG ratios in the crossing (green dots), the north transept (blue dots) and the nave (khaki dots).
Implications for the structural integrity of Notre Dame after the fire
This maximum temperature is important for safe and effective reconstruction. The findings by Raman paleothermometry established that the highest temperatures were around 1200 ˚C.
A temperature of 1200 °C is hot enough to melt the lead on the cathedral roof. The presence of resolidified flows of liquid lead on the cathedral walls confirms this. However, these temperatures are below 1740 ˚C and therefore not high enough to cause evaporation. Any lead pollution surrounding the Notre-Dame cathedral cannot be due to the direct evaporation of roofing lead during the fire and suggests contamination by aerosols.
High temperatures may also have affected the mechanical strength of limestone masonry. As a result of thermal stress, microcracks can form within the limestone from 300 ˚C. This can lead to an increase in porosity with a reduction in density and strength. Beyond 900 ˚C, solid limestone (CaCO3) decarbonates to produce powdery lime (CaO). The Notre-Dame fire frequently exceeded 1000 ˚C. Therefore, these thermoalterations could have ongoing effects on the limestone framework of Notre Dame.
Why do archaeologists use Raman microscopy to study ancient chars?
Archaeologists study ancient chars to understand the lives of prehistoric humans. In the search for early traces of fire domestication, chars indicate the presence of fire. Chars are solid residues resulting from the pyrolysis of organic matter. These could be charcoal, charred meat or charred fat. Analysis of these residues can reveal the type of fuel used and the eating habits of the ancient occupants of an archaeological site.
Raman microscopy is well suited to the study of archaeological chars. It is sensitive to the aromatic nanostructure of carbon compounds while being non-destructive. Raman analysis requires only a few μg of sample and has a spatial resolution below 1 µm.
Can Raman spectroscopy differentiate between charcoal and bone chars?
Raman spectroscopy is a promising tool for characterising carbonised fire residues in prehistoric hearths or fireplaces. Even when weathering has changed the characteristic morphologies of organic precursors, the vegetal or animal origin of chars can still be determined.
D. Deldicque and his co-workers developed a method for differentiating between weathered chars of vegetal or animal originiii. They noted that the carbonyl band at 1700 cm−1 is more intense in the Raman spectrum from weathered charcoal (vegetal origin). The same band is much weaker in the spectrum from a weathered animal char. In addition, the HD/HG ratio is often higher in the Raman spectra of weathered animal chars than in weathered charcoals. By plotting the intensity of the band at 1700 cm−1 as a function of the HD/HG height ratio, they could separate the Raman spectra from charcoal and animal chars into two distinct fields.

Speleofacts ring structure built by Neanderthal people in Bruniquel cave 176,500 years ago.
They then applied this method on two weathered chars from a hearth within the Bruniquel cave system. The Bruniquel caves are interesting as an archaeological site from Palaeolithic times. The caves are famous for annular structures made of broken stalagmites. These structures are around 176,000 years old and prove that early Neanderthals built elaborate structures.
Both amorphous chars from the Bruniquel caves have ambiguous globular morphologies, without any distinguishing vegetal or bone structures. Using the Raman spectra from these samples, they plotted the i1700/iG ratio as a function of the HD/HG ratio. The plot indicates that amorphous char 1 is an animal char and that amorphous char 2 is of vegetal origin, otherwise known as vitrified charcoal.
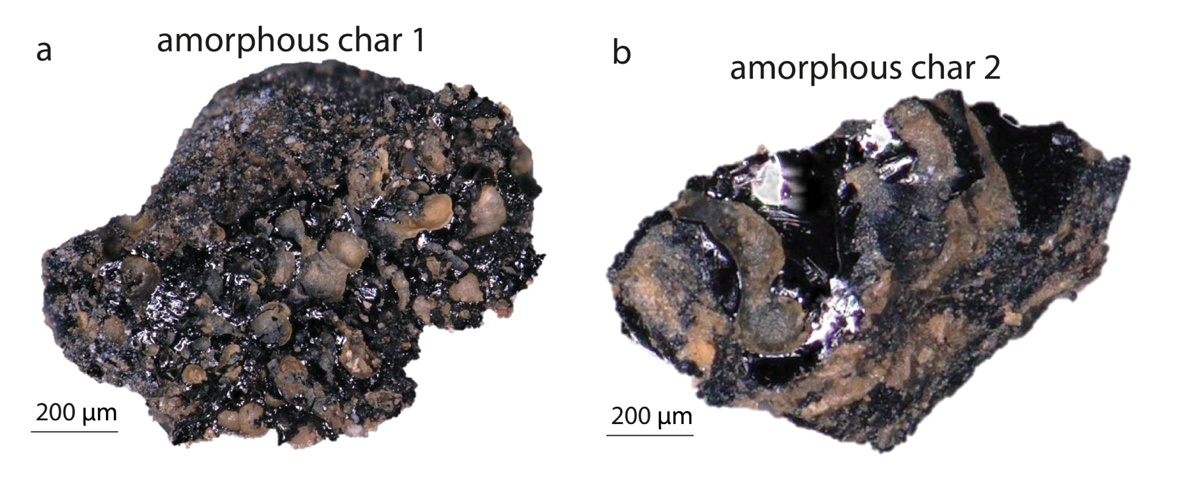 Amorphous chars found in a fireplace in the Bruniquel cave. The lack of specific morphology makes it difficult to visibly identify the precursors, which could be vitrified charcoals or charred fat or meat. (a) Amorphous char 1. (b) Amorphous char 2.
Amorphous chars found in a fireplace in the Bruniquel cave. The lack of specific morphology makes it difficult to visibly identify the precursors, which could be vitrified charcoals or charred fat or meat. (a) Amorphous char 1. (b) Amorphous char 2.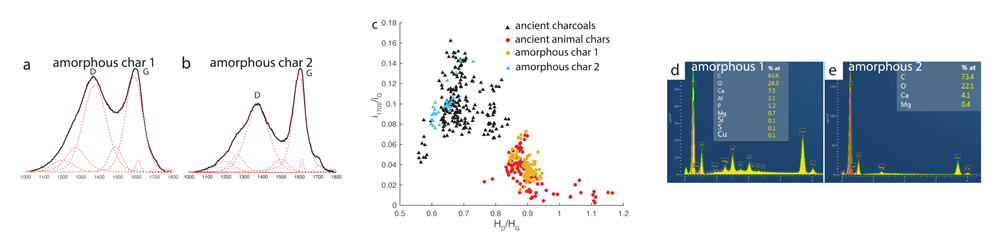 (a) Raman spectrum of amorphous char 1. (b) Raman spectrum of amorphous char 2. (c) Plot of Raman parameters placing amorphous char 1 in the animal field and amorphous 2 in the vegetal field. (d,e) Energy dispersive spectra of amorphous chars 1 and 2, respectively.
(a) Raman spectrum of amorphous char 1. (b) Raman spectrum of amorphous char 2. (c) Plot of Raman parameters placing amorphous char 1 in the animal field and amorphous 2 in the vegetal field. (d,e) Energy dispersive spectra of amorphous chars 1 and 2, respectively.To verify this result, the authors used scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS) to analyse both amorphous chars. The EDS result shows that amorphous char 1 contains phosphorus, calcium and sulphur which confirms an animal char. The EDS analysis of amorphous char 2 shows the presence of calcium and magnesium which confirms a vegetal precursor. In this way, Raman spectroscopy can discriminate between the animal or vegetal origin of ancient chars. This applies even when their characteristic morphology has been lost over time.
Raman analysis of chars for studying archaeological fire
Raman spectroscopy is ideal for studying the structure and chemistry of highly carbonized materials. This can provide clues not just about the material itself, but its precursors and the maximum carbonisation temperature. In the future, Raman measurements might shed even more light on humanity's past.
References
i A Raman – HRTEM study of the carbonization of wood: A new Raman-based paleothermometer dedicated to archaeometry, D. Deldicque, J.-N. Rouzaud, B. Velde, Carbon, Volume 102, 2016, p. 319-329.
ii Temperatures reached by the roof structure of Notre-Dame de Paris in the fire of April 15th 2019 determined by Raman paleothermometry, D. Deldicque, J.-N. Rouzaud, Comptes Rendus. Géoscience, Volume 352, 2020, no. 1, pp. 7-18.
iii Effects of oxidative weathering on Raman spectra of charcoal and bone chars: consequences in archaeology and paleothermometry, D. Deldicque, J.-N. Rouzaud, S. Vandevelde, M. Á. Medina-Alcaide, C. Ferrier, C. Perrenoud, J.-P. Pozzi and M. Cabanis, Comptes Rendus. Géoscience, Volume 355, 2023, p. 1-22.